Thirteen-year-old Tommy Fangboner had a broken heart. The freckle-faced Ellensburg boy with the engaging grin and buzz cut wasn't pining away after a pigtailed classmate. Tommy's heart was literally broken. He suffered from an inoperable condition that blocked the delivery of oxygen to his blood.
If—it was a big if—Tommy made it into adulthood, his quality of life would be marginal. His heart was already ballooning as it strained against the blockage.
The best course of treatment would be to surgically reduce the blockage, but back in Tommy’s day—the 1950s—such a feat couldn’t be done.
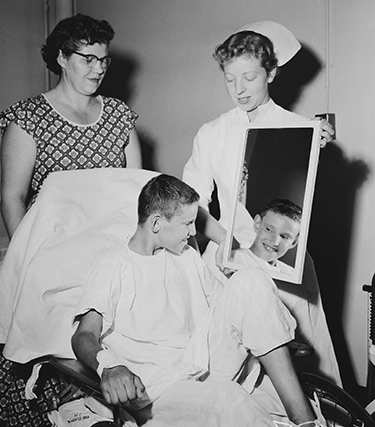
A nurse shows young Tommy Fangboner the results of a haircut shortly after his historic open-heart surgery. Photo courtesy of Seattle Post-Intelligencer Collection, Museum of History & Industry.
Across the country, surgeons were operating on the heart, but procedures were limited. It was nearly impossible to do surgery on a beating heart, but if you stopped the heart, the patient would die.
To repair a defect like Tommy’s, a surgical team would have to re-route the circulatory system while the surgeon cut into the heart to make repairs—what we now call open-heart surgery. If blood could be detoured around the heart, get oxygen and then return to the body, the heartbeat could be slowed or completely stopped for surgery.
As early as the 1930s, researchers were working on the prototype of the modern day heart-lung or bypass machine. In the 1950s, a University of Minnesota physician had begun a series of open-heart procedures using “living” heart-lung machines—the patient’s own mother or father. Surgeons would attach vessels to the parent’s groin area and re-route the child’s blood through the parent’s circulatory system before pumping it back into the child undergoing the cardiac repair.
Elsewhere in Minnesota, physicians at the Mayo Clinic were perfecting a mechanical pump to take over oxygenation of the blood during open-heart surgery. Early attempts had problems, but a team in 1955 had the first successful open-heart surgery in the world, heralding a new era. The “bypass machine” promised to revolutionize cardiac surgery.
***
Ellensburg of the 1950s was a rugged cattle town, best known for its annual rodeo. When the Fangboner family traveled to Seattle in June 1956, the UW School of Medicine was only 10 years old. But the family held out hope. UW Cardiologist Robert Bruce believed that Tommy would be a good candidate for an open-heart procedure, but no such surgeries had been tried outside of Minnesota.
Bruce knew that his UW colleagues were working on a new version of a bypass machine and looking for a potential first patient. Experimental Surgery Director K. Alvin Merendino, a young surgeon who had come to the UW from Minnesota, was leading a bypass machine team of surgeons, anesthesiologists, physiologists and bioengineers.
Merendino was a multi-talented physician who thrived in an era when the surgeon “did it all.” “I was a general surgeon,” says Merendino, now 91 and living in Seattle. “None of us specialized in those days. In my role, I generally took on the most complicated surgery cases, no matter what they were. I was interested in cardiac surgery and the work that was going on in Minnesota. My goal was to improve the oxygenator.”
Joining Merendino’s team was a young engineering student named Wayne Quinton who was still a couple of years away from completing his graduate work. Quinton, ’58, ran the instrument shop at the UW. He had many conversations with physicians who were seeking ways to better health care, and Quinton applied his engineering principles to try to solve these problems. This collaboration was the genesis of the UW’s Department of Bioengineering, which was established in the 1960s.
Over his career, Quinton created more than 30 innovative devices to help physicians better diagnose and treat patients. His most famous invention came as a result of his work with Medicine Professor Belding Scribner, when they designed and built a shunt to allow for long-term kidney dialysis.

Dr. K. Alvin Merendino performed the first successful open-heart bypass surgery in the western U.S. 50 years ago.
In 1956, Quinton was helping build a better heart-lung machine. By summer, the team felt they had developed an oxygenation device superior to the Mayo Clinic machine. Tommy’s would be the first open-heart surgery outside of Minnesota using a bypass machine.
On Aug. 1, 1956, Merendino and his team gathered in the operating suites on the 7th floor of UW’s de facto teaching hospital, the old King County Hospital, now known as Harborview Medical Center. A young resident named George Thomas, ’58, recalls that it was an uneventful surgery—the team had rehearsed this moment a hundred times, and there was no doubt in anyone’s mind that it was going to work.
“We were ready for this,” recalls Thomas. “We walked in and Merendino said ‘Are you ready boys?’ and we were.”
An Aug. 12, 1956, newspaper article recounted the groundbreaking surgery. ”An incision reaching from armpit to armpit was made and the heart was exposed. Two tube-like catheters were placed into the main veins leading into the heart, shutting off the blood flow. Pumps immediately began drawing out the blue blood and circulated it through the artificial lung, which added the correct amount of oxygen, at the same time removing waste carbon dioxide.
“The normal circulation back into the heart was blocked by the lower left valve. As steel and plastic quivered in emulating the duties of the body’s most delicate organs, Tommy’s blood coursed normally through his body. The heart was as still as death …”
For nearly half an hour, the mechanical heart-lung machine kept the young boy alive. Tommy Fangboner’s heart did not beat. In that half an hour, a three-inch incision was made into the right ventricle of Tommy’s heart and the extraneous muscle was cut away before the incision was closed. Doctors disconnected Tommy from the bypass machine, and his heart began to beat again.
The following morning, as he gazed out over Elliott Bay from his hospital room at Harborview, Tommy asked his nurses for a milkshake. He was looking forward to returning to his life in Ellensburg, his world of rodeos and baseball—never mind that he had made cardiac history; he was just a boy whose heart needed to be fixed.
Merendino later became chair of the surgery department. He last spoke to Tommy Fangboner about 15 years ago and says his first bypass surgery patient was still healthy and living a full life in eastern Washington.
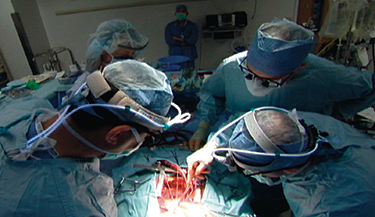
UW Surgeon Gabriel Aldea performs modern-day heart surgery. Image courtesy of UWTV.
Today’s chief of cardiothoracic surgery at the UW, Surgery Professor Edward Verrier, marvels at the work of Merendino and others. “Those were the days of the cowboy,” says Verrier. “You couldn’t do the things they did back then today—they were real pioneers. Merendino attracted physicians to the UW and Children’s who were good clinicians as well as good scientists. The work that Merendino did and the work of Dave Dillard and others had very significant implications for enhancing our ability to treat heart disease, and are standard practice in complex cardiac surgery.”
Merendino’s work was only the beginning of a long list of cardiac firsts or refinements by UW School of Medicine faculty. Surgery Professor David Dillard pioneered techniques that used hypothermia in the OR—by packing the body in ice, the patient’s temperature drops, slowing or suspending the heart while doctors operate. Professor Bruce developed the cardiac treadmill that is used routinely today to test cardiovascular function. Fellow UW Cardiologist Harold Dodge developed a method to test left ventricular function by measuring the volume of blood that is pumped with each beat of the heart. Both the treadmill and the technique for measuring blood volume are standards employed throughout the world today.
The most dramatic step after open-heart surgery came in December 1967, when South African surgeon Christian Barnard performed the world’s first successful heart transplant. The actual surgical procedure of transplanting a heart was only one dimension of a successful transplant—preventing the body from rejecting the “invading” organ would take monumental work in order to make heart transplant a viable therapy for end-stage heart disease.
In 1985, Medicine Professor Margaret Allen arrived at the UW to become chief architect of the region’s first heart transplant program, based at UW Medical Center. In the first year, the program—which now serves Wyoming, Washington, Alaska, Montana and Idaho—successfully transplanted 12 patients.
What once made worldwide headlines is now everyday surgery. Over the past 21 years, the UW Medical Center has performed more than 300 transplants; 70 percent of the patients have survived 10 years or longer. The largest stumbling block, however, is finding enough organs for the 90,000 patients nationwide who wait for this life-saving therapy.
***
If Tommy Fangboner walked in the doors of UW Medical Center today, he would have essentially the same surgery that was performed in 1956, according to George Thomas, the young resident who assisted Merendino on the historic case. The incision would have been vertical rather than "armpit to armpit" so that the ribs could be pulled apart to provide the surgeon with better access to the heart.
But Thomas, a practicing cardiovascular surgeon at Swedish Providence, thinks that in the future we will see less and less cardiac surgery. “We’ll be out of a job in 10 to 12 years,” he says with a laugh. “There are so many more non-invasive procedures that are changing how we treat heart disease.”
“The cardiac surgeon is like the canary in the coal mine,” adds Medicine Professor Larry Dean. “They are the first to notice the changes that improved non-surgical treatments have had on cardiac care. Statins, beta blockers and ACE inhibitors are changing the face of how we prevent and treat heart disease.”
In the early 1980s, physicians established a link between cholesterol and heart disease through a multi-city study that included the UW Since that time, new, powerful drugs to help control cholesterol are routinely used, such as statins and fibrates.
Other drug therapies have improved non-surgical treatment. Two decades ago, aspirin and morphine were the “heart drugs” of choice; today, drug-eluting stems—devices that keep the artery open while delivering powerful drugs to prevent plaque from adhering to the walls of the artery—have shown great promise.
Verrier and his colleagues perform intracardiac surgeries, transplants, bypasses and other life-saving and life-enhancing surgeries of the heart. Today, various assistive devices are also being used to treat cardiac patients. These tiny ‘machines’ help the heart beat more effectively and efficiently.
These devices are not implanted into the chest, but instead are placed in the heart through blood vessels in the leg. They can support patients during high-risk coronary interventions or during recovery from a major heart attack.
“We are now implanting Left Ventricular Assist devices or LVDs as a bridge to transplant, and in some cases, we are using the device as a permanent therapy,” says Verrier. “We have performed nearly 200 procedures in the last five years. There are some patients who have been using the devices successfully for almost three years.”
Other patients may be candidates for the tandem heart system, a device that actually sits on the patient’s thigh, with tubes inserted into the femoral artery. It pumps blood and can get patients through a crisis situation without surgery—this may be the closest we have come to an artificial heart, Verrier says.
Both Verrier and Dean believe that the next great strides in cardiology lie in research that is currently being conducted on the molecular and cellular level. Chuck Murry is a UW professor of pathology who is making great strides in stem cells and regenerative medicine, with a particular focus on building heart tissue from stem cells.
“Heart muscle cannot regenerate itself,” says Dean. ”And we just don’t have enough hearts for transplants. Murry is on the right track—looking at stem cells to regenerate tissue—and his work may be ready for human trials within the next three or four years.”
In addition, Verrier believes that surgeons will be looking at ways to transplant non-human hearts into people with end-stage cardiac disease. These hearts may one day be used to replace irrevocably damaged human hearts.
And if Tommy Fangboner should return to the UW 50 years from now, what might he expect?
Merendino is ever optimistic about what medicine will accomplish. He hypothesizes that one day the cardiac surgeon may actually remove the ailing heart and take it to the bench for repair before returning it to the body. “It is not out of the question,” he says. ”Advances in heart care have been quite dramatic.
“We all stand on the shoulders of those who have come before us—each physician making a new contribution. Within my lifetime I have seen such tremendous change—I never envisioned that medicine would be like this today. Who knows what it will be like in another 50 years? We can never underestimate the ingenuity of the human mind.”
And the beat goes on for father-daughter transplant recipients

Gordon and Terri Ochs received heart transplants at the UW Medical Center three days apart.
My husband, Gordy Ochs—a former Seattle firefighter and motocross racer—had always been extremely active. But in 1987, he was diagnosed with an asymptomatic heart problem. The doctors called it cardiomyopathy—his heart was enlarged and beating inefficiently, although he was not yet exhibiting all the symptoms of this debilitating heart condition. His cardiologist, Daniel Fishbein, a UW professor of medicine, told us that Gordy would one day become symptomatic. Once Gordy began gasping for a breath and showing other symptoms, his condition would rapidly deteriorate.
By spring of 1991, Fishbein’s prediction had come true. Gordy was hospitalized with full-blown heart failure and would remain in the hospital until a suitable donor heart could be found. If we did not find one, we had reached the end our options—in 1991, there were no devices to help the heart beat more efficiently.
In the sixth week of Gordy’s hospitalization, we were stunned when Gordy’s 29-year-old daughter, Terri, was admitted to the same cardiac intensive care unit with acute heart failure. After months of misdiagnosis, this mother of two was finally diagnosed with the same disease as her father—only her condition was much more critical.
Terri jumped over everyone else on the waiting list—including her father—to become the top candidate for the next available heart. Miraculously, within 48 hours, the transplant team was prepping Terri for a new heart. She had little time to think it over.
On May 31, Cardiac Surgeon Edward Verrier transplanted the heart of a Montana teen killed in a car crash into Terri. It was an amazing, unexpected event.
Although I was thankful for my stepdaughter’s good fortune, I knew the odds were against my husband making it through the summer. Months can pass between finding suitable donors for transplants. But just three days later, the family of a 30-year-old with a traumatic head injury said the magic words: “We want him to be an organ donor.” Cardiac Surgeon Margaret Allen and her team prepped my husband and transplanted him on June 3, three days after Terri. It was the first back-to-back, father/daughter heart transplant in medical history.
Now, 15 years later, both Gordy and Terri sometimes struggle with the “downside” of heart transplantation. They have a greater susceptibility to infection and side-effects from their medications. The drugs they must take for the rest of their lives are hard on their bodies, and their liver and kidney functions are constantly monitored. Rejection—even after 15 years—is always a possibility and is monitored by cardiologists who perform periodic heart biopsies. This downside is nothing compared to the alternative.
Several weeks after his transplant, Gordy asked Fishbein what he should expect from his new heart. What was the life expectancy? We knew the five-year statistics but we were optimistically looking ahead. “What about the 10-year survival rate?” Gordy asked hopefully. The good doctor smiled and said, “You tell me when you get there.”
This past July, Gordy and I attended the marriage of Terri’s daughter. It was a milestone that neither Gordy nor Terri thought they would ever make. With his new heart, there is no stopping Gordy. He snowmobiles, golfs, and still races—last year he won the regional championship for his age group in motocross.
Gordy and Terri are living proof that transplants work. Thank you to the amazing team from the UW Medicine Regional Heart Center; thank you from the bottom of all our hearts.
To find out more about organ donations in the Pacific Northwest, contact LifeCenter Northwest at www.lcnw.org.