

Ever since the first human cast an envious eye at a bird's ability to fly, people have looked to the natural world for ideas to imitate and shape to their own needs.
Today, armed with powerful computers, imaging devices and other advanced technologies, engineers and scientists from many disciplines have begun to take the closest look ever at how and why nature does what it does—at all levels, from individual molecules and cells to entire organisms.
That look will result in a revolution, say engineering professors at the UW. Just as biotechnology is changing the way we fight disease and advance our health, another “bio” discipline—called “biomimetics”—will change the materials we use to work and live.
“We are on the brink of a materials revolution that will be on a par with the Iron Age and the Industrial Revolution,” says Materials Science and Engineering Professor Mehmet Sarikaya. “We are leaping—we are not running—into a new era of materials. Within the next century, I think biomimetics will significantly alter the way in which we live.”
Biomimetics draws on some of the most powerful source material imaginable: hundreds of millions of years of evolution in which nature, slowly, painstakingly, has perfected or discarded creature after creature, adjusting and refining all life.
Reaching into nature’s vast trove of time-tested, unpatented ideas—some of the very processes that have enabled the world to tick on quite nicely, thank you, for as long as it has—these researchers think they can figure out how to make useful things from materials that not only are stronger, tougher and superior to what we have today, but that also don’t harm the environment or use excessive amounts of energy.
“Basically, we're stuck. The world needs lighter planes, lighter cars, lighter engines that can operate at much higher temperatures, and a host of other new materials.”
Mehmet Sarikaya, Materials Science and Engineering Professor
To this end, scientists at the UW College of Engineering and elsewhere have begun—barely, they concede—to take apart and analyze everyday marvels from the natural world: things as simple as how a spider spins its silk, a slug secretes its mucus, or an abalone grows its shell.
The payoff will not begin for a decade or two. But when it comes, brace yourself for what Newsweek last December, in an article describing biomimetics at the UW, termed “the next revolution in what the world is made of.”
Even at its infancy, the field’s possibilities are stunning:
How necessary is this revolution? Materials scientists say the world has taken petroleum-based plastics and fabrics about as far as possible. But the need for new, tougher, stronger, lighter and more energy efficient and environmentally sound materials is ever present.
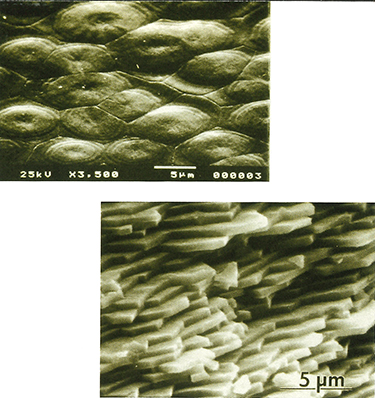
Bricks and mortar: At top, an electron microscope enlarges a section of an abalone shell 10,000 times, showing the brick-and-mortar architecture. At bottom, an electron microscope image of a fracture surface suggests that the shell is tough and strong. Photos courtesy of Mehmet Sarikaya.
“Basically, we’re stuck,” says Sarikaya, a pioneer in biomimetics. “The world needs lighter planes, lighter cars, lighter engines that can operate at much higher temperatures, and a host of other new materials. But today, we’re still working with the ceramic materials we’ve had for the past 25 to 30 years, for example, and metals that have been around for 100 years.”
UW Bioengineering Professor Christopher Viney half-jokingly puts it like this: “The challenge is to make things that are stiff and tough and have the right color and the right fatigue properties, that don’t cost anything, won’t hurt the environment and can be made out of beach sand.”
Viney, who also is an adjunct professor in materials science and a 1992 UW Distinguished Teaching award-winner, weaves his biomimetics research into lectures for incoming engineering students. First, he shows a slide of a helicopter made from Lego bricks. He asks the students to describe what they see. “They always respond the same way,” says Viney. “They either say it’s a helicopter, Lego bricks, gears or some such thing—but I never get the answer I’m trying to lead them to.”
Next, he flashes up a slide of a tractor and crane, also built from Lego bricks. “I tell the students, ‘Same Lego kit, exactly the same pieces. Different toys built from it.’ And then I go on to explain that what nature offers us is a set of building blocks: 20 amino acids. And from these, all the proteins in the world—the millions of different types of proteins necessary to sustain every form of life on earth—are manufactured. But there are only 20 types of blocks in the starter kit. Just 20.
“The idea is to bring nature into the repertoire of tools for inspiration and get ideas for how to proceed,” he continues. “Nature didn’t teach us to make those things, but there are some really neat starting materials lying around.”
Viney and Sarikaya are among approximately 500 biomimeticians in the United States—a field where the U.S. is the undisputed world leader. The goal, the two agree, is not only to understand nature’s way of doing things, but to do nature one better—and perhaps faster. “The traditionalists say, ‘If nature makes it, what’s new?’ ” says Viney. “That’s why I am very careful to say, we are selecting what we learn from nature—not trying to copy it exactly, because there would be no point in that.”
Currently, Viney’s main research interest is liquid crystals—substances suspended in a state somewhere between a solid crystal and a liquid. Millions of us wear artificial liquid crystals on our wrists: they make up the display screen for digital watches.
While liquid crystals may be commonplace in a watch, Viney and his colleagues surprised the scientific world when they wrote that silk secreted by silkworms and spiders owes its exceptional strength to temporarily becoming a liquid crystal. The 1991 report, published in Nature, was co-authored by UW graduate student Keven Kerkam, and colleagues from the U.S. Army Research, Development and Engineering Center.
“Now we have a gateway to understanding how to develop new technology. We have the blueprints nature used, and we can re-engineer them in our own land.”
Pedro Verdugo, Bioengineering and Biostructure Professor
The team found that as the golden orb weaver spider secretes its webbing, molecules in the droplets align themselves in rod-like structures—passing through an interim phase in which they take on a semi-ordered structure. The result is a material that, when solidified, can support far more weight for its size than steel.
By understanding how spider silk works, Viney believes we may someday use this information to improve bulletproof vests and build stronger suspension bridges, for instance.
Viney and others involved in biomimetics often speak of “hierarchical microstructures,” which are like a catalog of Lego constructions. The same components can be assembled and reassembled again and again in things that may be very different from one another.
How different? As unlike, say, as multilegged spiders and slimy slugs. Earlier this year, Viney, Bioengineering and Biostructure Professor Pedro Verdugo and UW graduate student Anne Huber became the first to report that there’s much more to the mucus highway secreted by common banana slugs than previously thought.
Though mucus research lacks mass appeal, it can shed light on a host of human health problems, including cystic fibrosis and other abnormalities in the reproductive and digestive tracts. To Viney and Verdugo, mucus is one of nature’s most remarkable substances. Slugs use it not only for transportation, but also for protection—enabling them, for instance, to creep harmlessly along the edge of a razor blade.
Verdugo, who has studied mucus in a variety of species for nearly two decades, and other researchers had thought that the microstructure of mucus was much like a randomly tangled web of spaghetti—its consistency dependent on the extent of tangling (the more tangled, the thicker). However, once released from the small granules in which it is stored inside the cell, slug mucus can swell up to 50 times its initial volume—so fast that researchers use the term “explosive.” In fact, mucus granules can expand from 10 to 500 microns in 15 to 20 milliseconds. And this had researchers stumped: How can something expand so fast, so efficiently, if it is as disorganized as a bowl of tangled spaghetti?
Now, much more is known, thanks to Viney and colleagues. Earlier this year, they showed that slug mucus is highly organized, not random. They found that before secretion, mucus molecules are stored in tightly packed, accordion-like bunches like jack-in-the-boxes. When secreted, the molecules—polymers with all the characteristics of liquid crystals—absorb water, expanding at high rates without involving high amounts of energy.
The tremendous range of expansion and condensation is what makes mucus one of nature’s “very best inventions,” says Verdugo. Understanding the structure is the key to the design of new and highly efficient polymer gels. “Now we have a gateway to understanding how to develop new technology. We have the blueprints nature used, and we can re-engineer them in our own land.”
While much research remains, Verdugo, Viney and others foresee many potential inventions coming from biomimetic materials based on mucus. For example, they might foster new drug delivery systems for cancer patients, pollutant traps in sewage treatment plants, and water-based lubricants. And if mucus makes such an ideal highway for slugs, light rail might someday be replaced by slime rail.
Another gastropod, the red abalone, absorbs much of Sarikaya’s interest. Over the course of its life, this seadwelling creature produces an impact-resistant shell so hard it can be run over by a truck without breaking. But if the shell does get damaged in the natural world, it heals itself—and later, when its occupant dies, the shell readily biodegrades. Finally, the abalone and similar organisms manufacture their shells at sea-water temperature using a readily available ingredient: calcium carbonate, a.k.a. chalk.
Broken down into components, abalone shells are relatively simple, explains Sarikaya, much like a single Lego brick. When assembled, however, the components—ranging in size from molecules to millimeters—function together to provide a material well worth mimicking: one that incorporates superb properties of toughness and strength.
It’s how these components, these bricks, are put together that makes the shell a miracle material. Using powerful electron microscopes, Sarikaya found beneath the shell’s outer portion a section consisting of extremely thin layers of laminate. It’s here that nature’s masons are at work, as flat bricks of calcium carbonate are set off from one another in typical brick-wall fashion. The “mortar” holding them together consists of small amounts of organic matter, mostly proteins and sugars.
Sarikaya and colleagues tested the abalone’s composite material and found that it has greater toughness and strength than conventional ceramics alone, owing to its brick architecture and the organic mortar. The next step is to unlock how the mollusk produces the proteins and sugar molecules that make up the mortar. Then they must discover how this material finds its way through the watery environment within the shell to the correct layer—and, once there, assembles itself exactly where it is needed.
To help break the secrets, Ilhan Aksay, formerly at the UW and now at Princeton, and Sarikaya enlisted the help of genetic engineers on the faculty of the UW School of Medicine: Medical Geneticist Clement Furlong and Microbiologist James Staley. The team hopes to invent genetically engineered organic “templates.” With these plans Sarikaya thinks he can grow technological materials the same way as an abalone produces its shell.
There even is the possibility of duplicating, perhaps using different materials, the abalone shell’s ability to self-heal. Does this mean it could never be destroyed? Not to worry, says Sarikoya; science also will come up with a way to switch this capability on and off.
This concept and other as-yet-unanswered questions about biomimetics lead even its chief proponents to caution that the future is not now. But it is coming, and at a far faster speed than it took technology to travel from Kitty Hawk to Cape Canaveral.
Ah, but if we are going to take things from nature, how can we be sure nature is right? “You can’t be,” answers Viney. “Nature has some ideas. That is, nature has optimized everything very nicely for what nature has to do. But this isn’t always ‘right’ for biomimicking.”
Feathers not only help birds fly, Viney wrote earlier this year, they also provide protection from bumps and bruises.
“Clearly, there is a useful suggestion here. But the materials engineer who hurries to cover his automobile with feathers might be better off wearing them himself.”