‘Super mice’ illustrate the power of genetic engineering
 To some people it’s the wave of the future in medicine. Others call it a new spin on an age-old practice. Still others believe it goes against the rules of Nature and of God.
To some people it’s the wave of the future in medicine. Others call it a new spin on an age-old practice. Still others believe it goes against the rules of Nature and of God.
This great debate is over the genetic manipulation of life. Scientists using technology discovered at the UW and other research universities are inserting bits of DNA from a person into a pig or a mouse. They say their research promises to unravel the mysteries of normal development and stop devastating diseases.
Others see such genetic tinkering as a natural—albeit high-tech—extension of crossbreeding techniques practiced by farmers since the dawn of agriculture. Some, however, believe it goes against nature or religious law to mess with the very “stuff of life.”
Whatever the viewpoint, a crucial piece of genetic engineering technology was born at the University of Washington and the University of Pennsylvania 13 years ago. UW Professor Richard Palmiter and Penn Professor Ralph Brinster proved that you could take part of a gene from one animal, transfer it to a different species, and then pass the trait along to the offspring of the altered species.
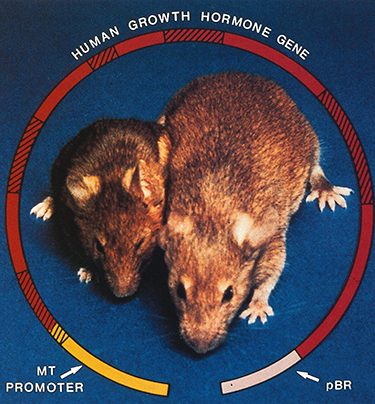
As often happens in basic scientific research, Palmiter and Brinster weren’t looking for a genetic breakthrough. They were trying to understand how cells read blueprints in genes to build living creatures. In the beginning, they weren’t planning to put a rat growth hormone gene into freshly fertilized mouse eggs. But when they did, the result was a virtual Mighty Mouse—some of the mice grew to be twice the normal size. When these “transgenic” mice had litters, many of the offspring also became Mighty Mice.
Over the years, transgenic mice have made a major contribution to understanding diseases such as Alzheimer’s, diabetes, chronic hepatitis and cancer. But Palmiter admits to some frustration with the slow pace of bringing other ideas to fruition, such as transgenic livestock. “It seems to me it’s taking forever” for that work to pan out, he says. Although transgenic tomatoes have hit the marketplace, “You can’t go out and buy transgenic pork chops,” he laughs.
That will change by the turn of the century. Already research institutes and business are forging ahead with genetic engineering on many fronts:
- The British biotech firm Imutran is growing pigs with a human gene so their organs could be transplanted into humans with less risk of rejection. This fall, the company reported that monkeys transplanted with pig hearts survived more than a month, rather than immediately rejecting the transplanted organs. Such technology could solve the worldwide shortage of donor hearts, kidneys and lungs. The company hopes to transplant pig hearts into people on an experimental basis starting early next year.
- “Molecular pharming” is turning animals into drug factories that produce important human chemicals. It’s the next step in a process that already has turned bacteria and yeast into “factories” for insulin and hepatitis B vaccine. For example, a genetically altered sow might make milk containing a human protein that regulates blood clotting. The pharmaceutical company would extract these substances from the animals and use them to prevent strokes.
- Companies are trying to breed pigs and cows that grow faster and produce leaner meat at slaughter, by adding growth hormone genes to their normal complement of DNA. It would be similar to “Flavr Savr,” the genetically altered tomatoes from Calgene Inc. already found at some supermarkets.
Dr. Richard Palmiter. Photo by Mary Levin.
Palmiter, whose specialty is biochemistry, and Brinster, whose specialty is veterinary medicine, earned international reputations as a result of their work. In 1994, the pair were awarded the highest honor bestowed by the French Academy of Sciences, the Charles Leopold Mayer Prize.
Being able to very specifically alter the genotype of animals and plants “will surely be noted as one of the major accomplishments of the 20th century,” says Biochemistry Chair Kenneth Walsh. “Many of the ramifications of this technology are just beginning to be realized,” he adds.
Palmiter, a lanky, soft-spoken man who sometimes sports a ponytail, has been at the UW for 21 years. Besides his role as an investigator with the Howard Hughes Medical Institute, Palmiter teaches biochemistry to nearly 300 undergraduates every year. His classroom experience shows. During an interview for this article, Palmiter frequently used the blackboard to illustrate his points, wielding red and black markers to indicate various parts of a gene, and to show how new genes are inserted into a strand of DNA.
Searching for metaphors to elucidate his work for non-scientists, he talks of splicing a snatch of a song into a tape recording or having a gadget (a gene’s regulatory region) that can find and copy a word (the structural part of a gene) on a tape with 100,000 words (the human genome).
Transgenic techniques are based on one fundamental fact. Genes have two main parts: the structural region that codes for a particular protein, and the regulatory region, or on- and off-switches that tell genes when, where and how effectively to do their job.
Palmiter and Brinster exploited the fact that the on- and off-switches from one gene can be attached to a completely unrelated gene and still have the same effect. This allows scientists to develop “designer genes.” Palmiter compares the early experiment to “taking a Chevy motor and seeing whether it would work in a Volkswagen body. When it does, it tells you the motor and chassis are separate elements and they can be swapped.”
Their revolutionary techniques grew out of work the pair were doing with the promoter, or on-switch, in the metallothionein (MT) gene. This gene contains the codes for a protein that binds the heavy metals zinc and cadmium.
They decided to take the on-switch from the MT gene and attach it to an unrelated gene. The pair chose a gene with codes for an easily measured enzyme (thymidine kinase). They then injected the composite gene into freshly fertilized mouse eggs, which are smaller than the dot above this typeset “i.”
“We were basically using mouse eggs as a test tube,” Palmiter explains. They discovered that cadmium, which normally turns on MT genes, now turned on the thymidine kinase gene in the mouse eggs. They decided to take the experiments further by transferring the fertilized eggs into a female mouse. It was a surprise when the gene became integrated into the DNA of one-third of the mice born.
“We were interested in metallothionein regulation and ended up developing transgenic techniques,” Palmiter says. Next, they used similar techniques to create super mice, by coupling the MT promoter with the rat growth hormone gene. The experiment, published in 1982 in the journal Nature, caught the attention of both the scientific and lay communities. Scientists saw the technique as a key to studying basic problems, such as gene regulation and medical problems like inherited disease. Entrepreneurs saw that it also had practical applications such as genetically engineered livestock.
“All sorts of applications come to mind when you realize the foreign gene is expressed and is affecting the physiology of the animal,” Palmiter says.
In further experiments, Palmiter and Brinster fine-tuned their understanding of a gene’s regulatory region, so that they could target a gene to be “expressed,” or do its work, only in a particular type of cell and only at a certain stage of development. They used the “deletion method” to understand where important regulatory regions lie.
The deletion method, Palmiter explains, is like pulling out parts of a car and seeing if it still works, or how differently it works, in order to understand whether the part is essential and just what it does. You wouldn’t have much luck running the vehicle without a carburetor, but you could still get where you’re going without the air filter. The trick was to whittle down a big chunk of DNA to only the bare essentials—to include the carburetor but not the air filter, so to speak.
Palmiter and Brinster have used their transgenic techniques to develop mouse models of several types of cancer and of human genetic disorders including chronic hepatitis, sickle cell disease, diabetes and Hirschprung’s disease, an intestinal malfunction. Other scientists have developed mouse models for maladies ranging from Alzheimer’s disease to Lou Gehrig’s disease.
The transgenic mice give researchers a living system for testing experimental treatments for some diseases. For example, Palmiter and Brinster have created mice with faulty livers and successfully implanted them with healthy mouse liver cells, which grew into completely new, functioning livers. In a cross-species experiment reported earlier this year in The Proceedings of the National Academy of Sciences, the pair showed it is possible to make rat livers grow and work right in mice bred to both develop liver failure and to have defective immune systems.
So how far off is the logical extension of this work—such as growing new human livers in people whose livers malfunction? Palmiter cautions that these experiments, while dramatic, are a long way from treatments that could cure people.
“The most important thing that comes from these mouse models is that you can see what you could expect if everything worked perfectly, because the genetics are very carefully controlled (in transgenic mice). Knowing it’s feasible is worth a lot,” he continues. “If you don’t know whether something is going to work in the first place, it’s hard to press ahead on a new therapy.”
The original model of transgenic mice mimicked dominant inherited diseases such as Marfan’s syndrome, which result from a single gene that has mutated, leading to a disruption in body chemistry. To mimic recessive inherited diseases such as cystic fibrosis, which generally occur because a gene isn’t operating, scientists developed “knockout” models, in which a gene is deactivated. A related technique changes existing genes by substituting a single nucleotide base for one already there, which allows scientists to precisely mimic genetic diseases like sickle cell anemia.
These techniques—developed during the ’80s by an army of scientists worldwide, not just Palmiter and Brinster—gave scientists the tools for adding, subtracting or changing any piece of genetic information that has been cloned. While today only 5 percent of mouse—or human—genes have been cloned, efforts are under way to map the entire human genome.
So what’s ahead? Palmiter continues to be interested in how the MT gene is regulated by heavy metals. His lab is also using transgenic techniques to study how the nervous system develops and functions. Using the knockout approach, they eliminate a gene essential to making the chemical messengers dopamine and norepinephrine as a way to explore the function of those neurotransmitters.
Because dopamine deficiency mimics diseases like Parkinson’s, this work may one day lead to better treatments—or a cure—for the degenerative disorder that strikes one in every 200 Americans. Or, like the early MT gene experiments, it could take off in a completely unexpected direction. Whatever the direction, one thing is certain. In this new era of genetic engineering, our lives will never be the same.
The heart of the matter: Who should decide when science crosses the line?
Does it make you queasy to think of pigs growing human-style hearts to be transplanted into humans? The research director at the English company Imutran, which hopes to perfect this transgentic technique, dismisses the ethical issues. “We have been using pig insulin for decades and pig heart valves,” he told Reuters in September. “You can’t accept the use of a pig heart valve and then object to using the whole heart to save someone’s life.”
If it could be done, should it be done? Who’s to decide these issues, anyway?
We the people, says Philip L. Bereano, professor of technical communication in the UW College of Engineering and past winner of the UW’s Outstanding Public Service Award.
Bereano wants the public at large to have a say in the direction we go with new technologies like genetic engineering. After all, he points out, much of the basic research that lays the groundwork for biotech and pharmaceutical products is paid for with tax dollars.
Bereano’s activism focuses on technology and public policy. He’s found that people who voice any concern about technology are often branded as “Luddites,” opposed to any progress on any front. He notes critics don’t always agree with each other. Some approach the issue from an animal rights point of view; others object on religious grounds; some voice a host of concerns about environmental consequences. “Calling them all Luddites means we don’t have to take them seriously,” he says.
People are right to have concerns, he insists. “We are not omniscient as to what will, in fact, happen when we alter genomes. Genetic manipulation is not like a child’s game of Legos or Tinkertoy.”
Bereano faults the education of scientists and engineers, which he believes is too technically focused. “There’s so little in their training and education today to give them an appreciation of the full social milieu.”
Too rarely, he believes, do scientists, government policy makers or corporate executives grapple with the ethical questions regarding technology and its implications for society.
True, broad-ranging public debates on complex issues can be slow and untidy, but tidiness isn’t what democracy is all about, Bereano contends. “It’s perfectly true that totalitarian states are far more efficient than democratic states. An empowered citizenry is unruly.”
If technology policy were set democratically, people would come to the table with different values and underlying assumptions, coloring even supposedly objective data, let alone political decisions like where research dollars should go, and how research results will be applied to the real world. None of that bothers Bereano. In fact, he delights in the notion of public squabbles over issues of such import.
“We need to acknowledge those differences,” he argues. “What we have done instead is make believe they don’t exist as a way to move ahead to get things done. I think that’s behind a lot of the cynicism surrounding government and other institutions now.”
Drawing the line “between socially permissible and unsavory activities,” is no easy task, he readily admits. “But the essence of being human is wrestling with these problems.”